The molecule Propane, also known as C3H8, is a linear molecule. C3H8 contains three carbon atoms, and eight hydrogen atoms. Its Lewis Structure is:
There is no single central atom for the molecule, but there are three carbons. The middle carbon has two hydrogens bonded to it, while the two end carbons have three hydrogens bonded. C3H8 is a
linear molecule because it has no specific central atom. Since the molecule is linear, it has 180 degree bond angles.
Below is a ball and stick model of Propane:
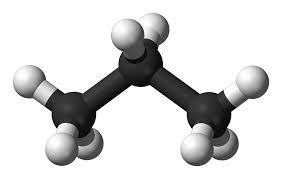

Everything said here is true. Due to the structure of the molecule and the equal distribution of atoms and electrons, the molecule has to be nonpolar. Thus the only force of attraction applicable to this molecule is London Dispersion Forces. However, I disagree whether this molecule is linear. As the 3-D models show, each of the carbon atoms bonded to one or two of the other carbon atoms and the hydrogen atoms make Tetrahedral shapes instead of linear. So maybe the central atom is the carbon, each of he carbon atoms being their own central atom, do to the correlation of shape and structure between the carbon atoms and the hydrogen atoms. Thus, I disagree that the shape of the propane molecule is linear.
ReplyDeleteAll in all, the appearance of the blog is very good. Although there are colors that sort of meld together and make the page pop out less, the blog is neat, concise, clear, and easy to follow. This appears to be a very structured blog.
ReplyDeleteThis is the molecule of propane. I see the hydrogen atoms attached to the carbon atoms and the carbon atoms attached to the hydrogen atoms. This is an accurate representation of the propane molecule.
ReplyDeleteI agree with you saying that the molecule is linear because there is no clear central atom. As far as we have been taught, in this case there is no central atom but according to the 3D representation, there is a distinct shape to it. Blake does make a good point in saying that maybe the Carbon atoms are there own central atoms. This idea would be beyond our learning though.
ReplyDeleteAlthough the bonds are shown in the Lewis Structure model, there are no representations of the polarity of the specific bonds or arrows showing that.
ReplyDeleteOverall, the blog looks very appealing and the pictures add a nice aid to what you had to say. There is not too much information and I feel I know enough about the molecule. Very nice job.
ReplyDeletemeh
ReplyDelete